The concept of regenerative medicine is based on the functional recovery of normal (body) function by replacing or regenerating cells, tissues or organs. To achieve this, natural mechanisms of the body are embraced. Adult stem cells intended for regenerative purposes reside within every mature human being. Adult mesenchymal stem cells are endogenous precursor cells that can specialize into various cell types to replace damaged tissue whenever necessary. Furthermore, they possess anti-inflammatory properties and produce cellular messengers (cytokines and growth factors) that stimulate the body’s own regeneration and repair processes. Due to their ability to facilitate a holistic healing process, adult mesenchymal stem cells are of major interest for our clinical research.
OXACELL® AG - regenerative therapies utilizing adult, mesenchymal stem cells
The Production is performed by our highly trained expert team of scientist and physicians in our GMP-certified production facility in accordance with the national and international authorities (clean room, process, equipment).
The extraction of highly potent mesenchymal stem cells from adipose tissue is a high-tech procedure utilizing state-of-the-art technology.
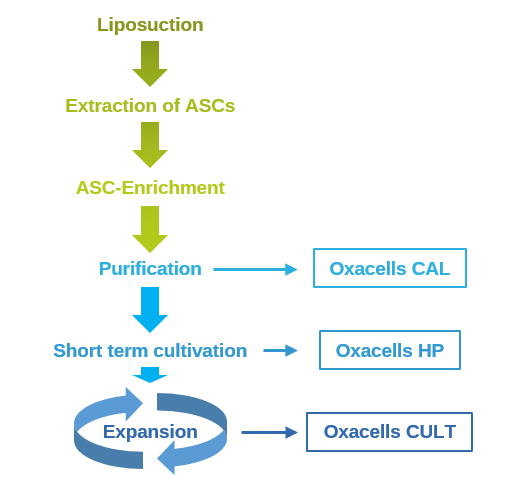 First, adipose tissue is harvested via liposuction by our specially trained physicians in one of our collection centers under local anesthesia. The risk associated with general anesthesia is omitted. Subsequently, the stem cells are being isolated under sterile conditions as per a standardized, well-established and tested procedure. For our product Oxacells CAL, the stem cells are subsequently mixed with a small part of the extracted adipose tissue. As a result of this enrichment, the properties of Oxacells CAL are drastically improved when compared to the conventional fat cell transplantation. Further purification (short-term cultivation) of the extracted stem cells results in Oxacells HP (high purity). HP is a highly potent medicinal product for the treatment of osteoarthritis in various joints. Our latest development is Oxacells CULT, a platform technology intended for allogenicenic therapies. Due to the excellent proliferative capacity, ASCs can be expanded (almost) indefinitely. This facilitates the medical treatment of thousands of patients with a single liposuction from a single donor. In comparison to similar products, our carefully balanced formulation (patent pending) greatly extends the shelf-life of CULT at 4°C. This allows worldwide supply without the need for additional manipulation (thawing/ elimination of cryoprotectants etc.) at the site of treatment.
First, adipose tissue is harvested via liposuction by our specially trained physicians in one of our collection centers under local anesthesia. The risk associated with general anesthesia is omitted. Subsequently, the stem cells are being isolated under sterile conditions as per a standardized, well-established and tested procedure. For our product Oxacells CAL, the stem cells are subsequently mixed with a small part of the extracted adipose tissue. As a result of this enrichment, the properties of Oxacells CAL are drastically improved when compared to the conventional fat cell transplantation. Further purification (short-term cultivation) of the extracted stem cells results in Oxacells HP (high purity). HP is a highly potent medicinal product for the treatment of osteoarthritis in various joints. Our latest development is Oxacells CULT, a platform technology intended for allogenicenic therapies. Due to the excellent proliferative capacity, ASCs can be expanded (almost) indefinitely. This facilitates the medical treatment of thousands of patients with a single liposuction from a single donor. In comparison to similar products, our carefully balanced formulation (patent pending) greatly extends the shelf-life of CULT at 4°C. This allows worldwide supply without the need for additional manipulation (thawing/ elimination of cryoprotectants etc.) at the site of treatment.
The production of medicinal products is under strict supervision of the regulating authorities. OXACELL® AG holds a manufacturing license according §13 AMG for two autologous products (Oxacells CAL and HP) as well as one allogenic product, Oxacells CULT. The stem cell products are manufactured at our GMP-certified production site (clean room, processes, equipment) involving comprehensive quality control tests in accordance with the national and international authorities.
We cooperate with competent partners such as the Rostock University Medical Center, University Hospital Magdeburg and the University Medical Center Hamburg-Eppendorf to develop concepts for individual therapies. In this setting, Oxacells stem cell products are already utilized in individual healing attempts (compassionate use) – to date with promising results.