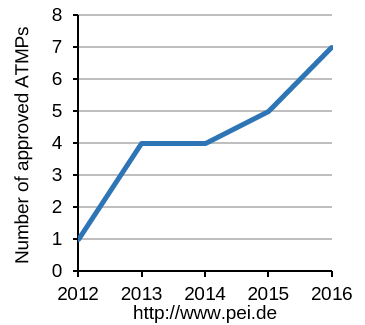
 Innovative therapies based on stem cells are on the rise worldwide. Due to tremendous efforts in basic research, our insight into the biological function and operation of our body increases daily. OXACELL® AG goal is the utilization of this knowledge for the development novel therapies (ATMPs, advanced therapy medicinal products). The steady increase in approved ATMPs for the treatment of various disease worldwide clearly supports the excellent medical potential of these new approaches.
Innovative therapies based on stem cells are on the rise worldwide. Due to tremendous efforts in basic research, our insight into the biological function and operation of our body increases daily. OXACELL® AG goal is the utilization of this knowledge for the development novel therapies (ATMPs, advanced therapy medicinal products). The steady increase in approved ATMPs for the treatment of various disease worldwide clearly supports the excellent medical potential of these new approaches.
The term ATMP includes gene therapy medicinal products, somatic cell therapy medicinal products as well as tissue engineered products. Stem cell-based medicinal products belong to the ladder category.
Adult stem cells are considered the bearer of hope for regenerative medicine.
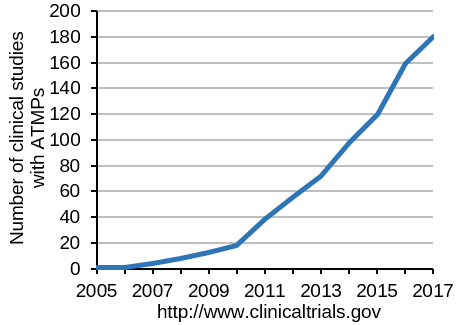 Intensive research is aimed at the medical application of stem cells for the renewal of old or diseased tissues. As a matter of fact, a large amount of scientific publications already demonstrated the regenerative and immune-modulatory properties of adipose-derived stem cells (ASCs). Moreover, more than 200 clinical studies using ASCs are conducted worldwide. The indications are as diverse as the patients involved.
Intensive research is aimed at the medical application of stem cells for the renewal of old or diseased tissues. As a matter of fact, a large amount of scientific publications already demonstrated the regenerative and immune-modulatory properties of adipose-derived stem cells (ASCs). Moreover, more than 200 clinical studies using ASCs are conducted worldwide. The indications are as diverse as the patients involved.
In 2015, the first stem cell-based ATMP was approved in Europe. This product is designated for the restoration of the eyesight after corneal injuries, demonstrating the enormous performance of modern stem cell therapies. Furthermore, this example impressively illustrates the feasibility to design clinical therapies based on stem cells according to the pharmaceutical standards.


